
HL Paper 1
Which combination of properties is correct?

Which group of ions and molecules has delocalized electrons in all the species?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{CO}}{{\text{O}}^ - }\) and \({{\text{O}}_{\text{3}}}\)
B. \({\text{NO}}_{\text{3}}^ - {\text{, NO}}_{\text{2}}^ - \) and \({\text{C}}{{\text{O}}_{\text{2}}}\)
C. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{, CO}}_{\text{3}}^{{\text{2}} - }\) and graphite
D. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{, CO}}_{\text{3}}^{{\text{2}} - }\) and \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
Which species contain dative covalent bonds?
I. CO
II. \({\text{N}}{{\text{H}}_{\text{3}}}\)
III. \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which sequence has the molecules in order of increasing nitrogen-nitrogen bond length?
A. \({{\text{N}}_{\text{2}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
B. \({{\text{N}}_{\text{2}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{2}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
C. \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{2}}} < {{\text{N}}_{\text{2}}}\)
D. \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{2}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} < {{\text{N}}_{\text{2}}}\)
Which substance has the following properties?
• Low melting point
• Very soluble in water
• Does not conduct electricity when molten
A. Glucose, \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\)
B. Silicon dioxide, \({\text{Si}}{{\text{O}}_{\text{2}}}\)
C. Sodium chloride, NaCl
D. Tetrachloromethane, \({\text{CC}}{{\text{l}}_{\text{4}}}\)
Which metal has the strongest metallic bonding?
A. Na
B. Mg
C. Al
D. Ca
Which compounds have an ionic lattice structure in the solid state?
I. Silicon dioxide
II. Sodium fluoride
III. Ammonium nitrate
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which diagrams can be used to represent the Lewis (electron dot) structure of boron trifluoride?

A. I and II only
B. I and III only
C. II and III only
D. I, II and III
What is the difference between the strength and the length of the carbon-oxygen bond in butanal and in butan-1-ol?
A. The bond in butanal is stronger and longer than in butan-1-ol.
B. The bond in butanal is weaker and shorter than in butan-1-ol.
C. The bond in butanal is weaker and longer than in butan-1-ol.
D. The bond in butanal is stronger and shorter than in butan-1-ol.
What is the formula of calcium nitride?
A. \({\text{C}}{{\text{a}}_{\text{3}}}{{\text{N}}_{\text{2}}}\)
B. \({\text{C}}{{\text{a}}_{\text{2}}}{{\text{N}}_{\text{3}}}\)
C. \({\text{Ca(N}}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{2}}}\)
D. \({\text{Ca(N}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)
Which molecule has an octahedral shape?
A. SF6
B. PCl5
C. XeF4
D. BF3
The Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) is given below.
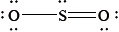
What is the shape of the \({\text{S}}{{\text{O}}_{\text{2}}}\) molecule?
A. Bent (V-shaped)
B. Linear
C. T-shaped
D. Triangular planar
Which correctly lists butane \({\text{(}}{M_{\text{r}}} = {\text{58)}}\), propanone \({\text{(}}{M_{\text{r}}} = {\text{58)}}\), propan-1-ol \({\text{(}}{M_{\text{r}}} = {\text{60)}}\) and propan-2-ol
\({\text{(}}{M_{\text{r}}} = {\text{60)}}\) in order of increasing boiling point?
A. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}} < {{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\)
C. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
D. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\)
What is the correct order if the compounds are arranged in order of increasing boiling point?
A. \({\text{C}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{Si}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{Si}}{{\text{H}}_{\text{4}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{Si}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{4}}}\)
D. \({\text{C}}{{\text{H}}_{\text{4}}} < {\text{Si}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
Zinc metal contains metallic bonding. Which is the best description of a metallic bond?
A. The electrostatic attraction between a pair of electrons and positively charged nuclei.
B. The electrostatic attraction between oppositely charged ions.
C. The electrostatic attraction between a lattice of positive ions and delocalized electrons.
D. The bond formed when one atom provides both electrons in a shared pair.
What is the bond angle in the \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) ion?
A. 104°
B. 107°
C. 109°
D. 120°
Which molecule contains a dative covalent (coordinate) bond?
A. HCN
B. \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\)
C. \({\text{C}}{{\text{O}}_{\text{2}}}\)
D. CO
How many electrons form the carbon–oxygen bond in methanal, HCHO?
A. 2
B. 4
C. 8
D. 12
Four identical sealed containers are prepared each containing \({\text{10 c}}{{\text{m}}^{\text{3}}}\) of an organic compound and at the temperature shown below. Which container will have the highest vapour pressure?

How many atoms is each carbon directly bonded to in its allotropes?

A solid has a melting point of 1582 °C and does not dissolve in water. It does not conduct electricity in the molten state. What type of structure does the solid have?
A. Ionic
B. Metallic
C. Giant molecular
D. Simple molecular
Which combination describes the bonding and structure in benzoic acid, C6H5COOH?